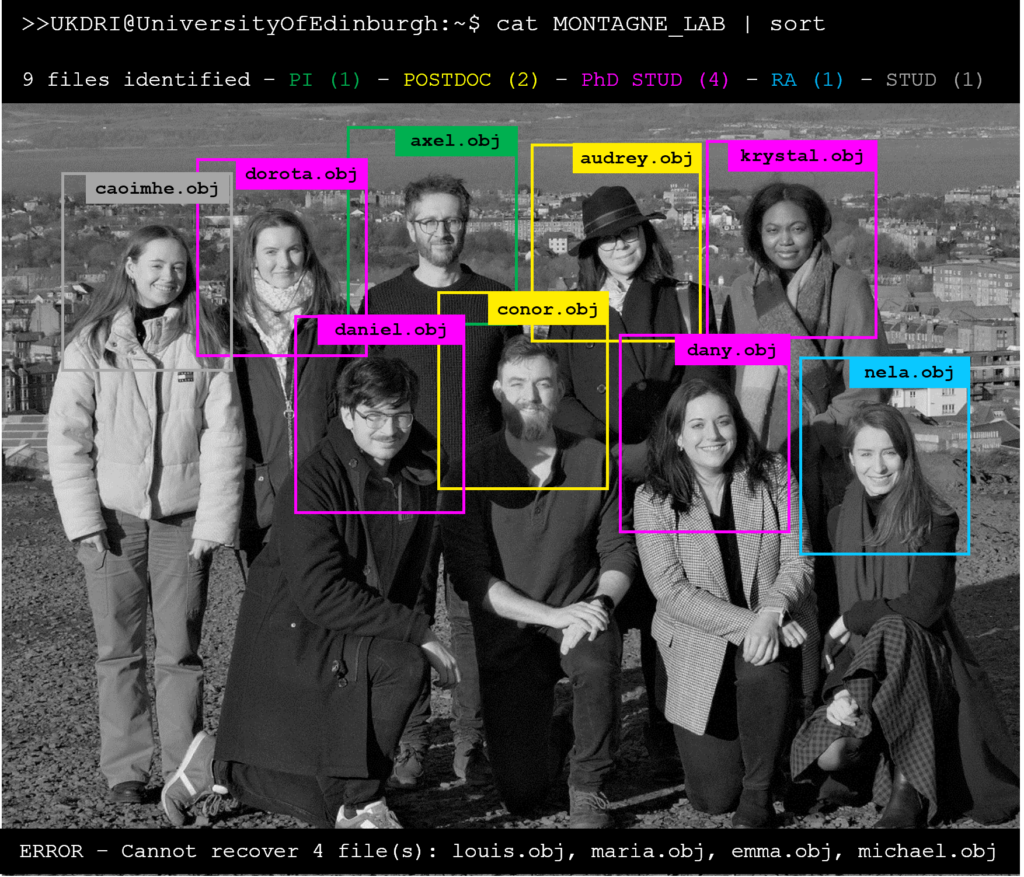

Axel Montagne, Ph.D.
Principal Investigator (axel.montagne@ed.ac.uk)
Axel joined the UK DRI at Edinburgh in December 2020. He completed his PhD degree at the University of Caen Normandy (France) in 2012, followed by a postdoctoral training at the University of Southern California (USC) in Los Angeles from 2013 to 2016. Axel rapidly became Assistant then Associate Professor at USC in 2016 and 2020, respectively. His carrier has been focusing on how cerebrovascular dysfunctions contribute to neurodegeneration and dementia in both animal models and humans. In this UK DRI project, he combines molecular approaches with rodent non-invasive imaging, particularly MRI, PET and two-photon microscopy, to study the causes and effects of blood-brain barrier (BBB) dysfunction, with a particular focus on the pericyte-endothelial crosstalk, in the context of neurodegenerative diseases. In his spare time, Axel enjoys spending time with his family, playing with his son, playing guitar, hiking, and all sorts of sports activities including football, basketball, and tennis.

Conor McQuaid, Ph.D.
Postdoctoral Researcher & Lab Manager (conor.mcquaid@ed.ac.uk)
Conor joined the Montagne lab in January 2022. He completed his undergraduate in Biomedical Sciences at Queen’s University Belfast, his Master of Research at the University of Liverpool, and his PhD in Neuroscience and Nanotechnology at the Open University with Midatech Pharma®. His career has focused on the cerebrovascular system and specifically the role of the blood-brain barrier and its part in hindering drug delivery and its contribution to neurodegenerative disease. He has worked with culture models, including primary, immortalized, & iPSC-based systems. As a Postdoctoral Research associate at the University of Rochester, he has worked on the glymphatic system and its role in disease, and SARS-CoV-2 and its interaction with the cells of the neurovasculature unit, in both in vitro and in vivo models. In the Montagne lab, Conor is investigating the crosstalk between pericytes and endothelial cells, and the link between the immune system and BBB dysfunction in both health and disease. Conor enjoys spending time with his fiancée and family, hiking and going on adventures, and playing with any and all dogs.

Audrey Chagnot, Ph.D.
Postdoctoral Researcher (audrey.chagnot@ed.ac.uk)
Audrey joined the Montagne lab in February 2022. She completed her PhD in the Pathophysiology and Imaging of Neurological Disorders team (INSERM U1237) at the University of Caen Normandy, France. She developped Magnetic Resonance Imaging protocols to study the glymphatic system in mice. In the Montagne lab, Audrey is studying how an impaired endothelial-pericyte crosstalk can trigger Brain Interstitial Fluid Accumulation Syndrome (BIFAS) using cutting-edge brain imaging technology. In her free time, Audrey enjoys reading encyclopaedias, crafting amulets, and writing short novels.

Dorota Stefancova
PhD Student (D.Stefancova@sms.ed.ac.uk)
Dorota joined the Montagne lab in January 2022 to complete her Neuroscience studies at the University of Edinburgh. She then stayed as a Research Assistant for a few months. She is going to start her PhD in October 2022 and will be investigating the role of different brain pericyte subtypes in health and disease. She also previously worked with pericytes and mesenchymal stromal cells in mice and humans. In her free time, Dorota enjoys hikes, baking, and reading.

Krystal Laing
Wellcome Trust Translational Neuroscience PhD Student (k.k.laing@sms.ed.ac.uk)
Krystal joined the Montagne Lab in September 2022 as a Wellcome Trust Translational Neuroscience PhD student. She is interested in underlying mechanisms of vascular contributions to dementia and investigating the role of Apolipoprotein E Ɛ4 variant in blood-brain barrier (dys)function. She completed her undergraduate in Neuroscience at Trinity College, Hartford, and her Masters in Neuroscience at the University of Hartford, USA. She has been a research assistant in neuroimaging studies of small vessel cerebrovascular disease in dementia with Prof. Adam Brickman at Columbia University, USA. In her spare time, Krystal enjoys writing in coffee shops, playing with her dog, visiting museums, theatres, and the cinema.

Dániel Réti
PhD Student (d.reti-1@sms.ed.ac.uk)
Daniel joined the Montagne lab in October 2022. He completed his undergraduate degree in Physics at Budapest University of Technology and Economics, Hungary. He earned his master degree in medical physics at University of Galway, Ireland in 2021. His focus was on medical imaging, during his studies. He contributed to projects of this field, like fMRI and phase contrast CT research. After his studies he started working for Mediso, which company installed two new preclinical imaging devices in Edinburgh in 2022. During his PhD research, he is finding way to optimise PET, MRI and CT quality control protocols, focusing on cardiac and brain imaging. His other PhD co-supervisors are Drs. Adriana Tavares and Maurits Jansen. In his free time he enjoys analogue photography, improve acting, and different kinds of sports.

Nela Fialova
Senior Research Assistant (nfialova@exseed.ed.ac.uk)
Nela joined the Montagne lab in January 2023 as a Research Assistant. She helps with smooth running of the lab, optimizing protocols, analyzing collected data and assisting with the MRI studies. She completed her undergraduate degree in Neuroscience with Psychology at the University of Aberdeen, followed by a Master by Research degree in Integrative Neuroscience at the University of Edinburgh. She continued her Master’s thesis project as a Research Assistant in the Horsburgh lab investigating the disruption of the neuro-glial-vascular unit in cerebral small vessel disease, particularly focusing on the role of pericytes in haemodynamic regulation. In her free time, Nela likes to dance, bake, read, hike, and play games.

Solvig Becker
Research Assistant (sbecker@ed.ac.uk)
Solvig joined the Montagne lab in October 2025 as a Research Assistant. She helps with the smooth running of the lab, colony management, data analysis, and various in vivo experiments. She studied at Cardiff University, gaining her Integrated Master’s degree in Biomedical Sciences before moving to Edinburgh to work as a Research Assistant at the Centre for Reproductive Health, looking at the role and ontogeny of the immune system during embryonic development. In her free time, Solvig enjoys reading, crafting, and playing the piano and trombone.

Ogochukwu Uweru
Postdoctoral Researcher (ouweru@ed.ac.uk)
OJU joined the Montagne Lab in December 2025. He earned a BSc degree in Human Physiology from the Ahmadu Bello University, Zaria and a MSc degree in Physiology from the University of Lagos, both in Nigeria. He completed his PhD in Neuroscience in Dr. Eyo’s lab in the Centre for Brain Immunology and Glia in the Department of Neuroscience at the University of Virginia, USA. During his doctoral work, he discovered that P2RY12 perturbation disrupts microglial-neuronal interactions with sexually biased behavioural consequences. He also contributed to studies identifying P2RY12 as a key regulator of microglial suppression of neuronal hyperexcitability and of microglial interactions with the brain’s capillary network. He then had a brief postdoctoral fellowship in Dr. Mishra’s lab at the Jungers Centre for Neurosciences Research in the Department of Neurology at Oregon Health & Science University, where he used Cre-lox genetics, electrophysiology, differential interference contrast microscopy, and calcium imaging to examine whether astrocytic mGluR5 drives neurovascular dysregulation following ischemic stroke. In the Montagne lab, he aims to uncover the mechanisms governing microglia-pericyte physical interactions and to determine how these interactions influence endothelial functions and cerebral blood flow in health and in dementia. In his free time, he enjoys deep, meaningful conversations, a good book, and unhurried walks.

Marina Romero Bernal
PhD Student (mromero-ibis@us.es)
Marina joined the Montagne Lab in January 2026 as a visiting PhD student for a short research stay through April 2026. She is interested in preventive strategies for neurovascular disease, focusing on blood-brain barrier (dys)function and the potential of polyphenols, particularly those from Salicornia ramosissima. Her doctoral work at the University of Seville, based at the Instituto de Biomedicina de Sevilla (IBiS), focus on developing an in vitro ischemic stroke model using blood-brain barrier spheroids. She completed her undergraduate in Biology at the University of Seville and a MSc in Health Biotechnology at the Pablo de Olavide University, Spain. In her spare time, Marina enjoys dancing flamenco, practising yoga, reading, and spending time with family and friends.

Anthea Choi
UG (C.W.Choi@sms.ed.ac.uk)
Anthea is an undergraduate pharmacology student joining the lab as an intern to complete her project on blood-brain barrier impairment in mouse models with pericyte depletion. She has a strong interest in neuroscience and hopes to explore research areas related to vascular dementia. Outside the lab, Anthea enjoys listening to music and learning new languages on Duolingo (1000+ day streak in Portuguese)!

Arwen Kennedy
UG (A.Kennedy-16@sms.ed.ac.uk)
Arwen joined the Montagne lab in January 2026. She is an intercalating Medical Student who is in her third year in Edinburgh University. Her intercalating degree is Surgical Sciences. She is doing her dissertation project within the lab, working with Conor on his research into the crosstalk between endothelial cells and pericytes as well as the link between the immune system and BBB dysfunction. In her free time Arwen enjoys going walking, cooking, or a good movie night. She also enjoys scuba diving in the sea lochs on the West coast of Scotland.

Katie Gregg
UG (K.Gregg@sms.ed.ac.uk)
Katie joined the Montagne lab in January 2026 as a BSc Biomedical Sciences student at the University of Edinburgh to complete her undergraduate research project. Her research focuses on VCAM-1 expression during vascular inflammation and investigating the influence of a novel compound which may inhibit VCAM-1 expression. In her free time, Katie enjoys travelling, cycling, and playing the guitar.

Louis the duck 🐥
Anxiety Mascot Plush (louistheduck@ed.ac.uk)
Louis the duck, quacked his way to our Montagne lab family in March 2024. His primary role within the lab is to spread happiness and alleviate stress, during those challenging moments when experiments don’t quite go as planned. He adores being snuggled and bringing calmness to even the most frazzled scientists.
Alumni

Mathieu Bellal
MD-PhD Student (bellal-m@chu-caen.fr, bellal@cyceron.fr)
Mathieu joined the Montagne lab in November 2024 as an academic visitor in an international PhD mobility. He completed his medical studies in Caen (Normandy, France), followed by a specialisation in clinical haematology obtained in 2019. He also obtained a complementary specialisation in Intensive Care Medicine in 2023. After his residency, he worked for 4 years in the Intensive Care Unit as Former Head of Clinic of the Universities, Assistant of the Hospitals of Caen. In parallel to his clinical career, he obtained a Master in “Biology-Health, signalling and functions in pathological conditions” at the ED 497 NBISE University of Normandy – INSERM UMR-S 1237 PhIND in 2019 (Professor Denis Vivien). He is currently a 4th year PhD student and his fundamendal and preclinical work focuses on acute neuroinflammation in the brain and on the role of brain mast cells in neurovascular diseases.

Clara Chuzeville
UG (clara.chuzeville@gmail.com)
Clara completed a Bachelor degree in Biological Engineering at the Claude Bernard University in Lyon (France), following a Medical Biology & Biotechnology pathway. Clara is an undergraduate student in Neuroscience, and next year she will begin a Master degree in Neuroscience. Her goal is to pursue a PhD and work in research, focusing on neurodegenerative diseases. She wants to contribute to the development of treatments that can improve the lives of people affected by these conditions. Outside of her academic interests, she enjoys hiking and exploring new places. She also plays volleyball and has studied music, so she spends some of her free time playing instruments and making music.
Khaula Elsaye
MSc Student (K.Elsaye@sms.ed.ac.uk)
Khaula is a Master’s student in Integrative Neuroscience and joined the Montagne lab after completing her undergraduate degree in Neuroscience at the University of Edinburgh. Her initial project focused on the blood-brain barrier, and she is now investigating whether large blood vessels in the brain lose their smooth muscle cell coverage during ageing. She is excited to further develop her research skills and contribute to the lab’s work. In her free time, Khaula enjoys horse riding and spending time with family and friends.

Ellen Campbell-Lendrum
Undergraduate (E.G.Campbell-Lendrum@sms.ed.ac.uk)
Ellen is a final year Neuroscience undergraduate and joined the Montagne lab in January 2025. She is English and French. She hopes to gain experience of working in a lab environment working on cutting edge research and looks forward to getting to know the other lab members. In the future, she aspires to focus on neurodegenerative or neuropsychiatric diseases and enhance their treatments, or to contribute to global and public health initiatives. In her free time, Ellen enjoys spending time with friends, reading, watching films and going to the gym.

Natasha Himsworth
Undergraduate (N.Himsworth@sms.ed.ac.uk)
Natasha joined the Montagne lab in January 2025 as a BSc Medical Sciences student at the University of Edinburgh to undertake her undergraduate research project. Her research focuses on the role of endothelial dysfunction in neurodegenerative diseases and ageing. Outside the lab, Natasha enjoys spending time with family and friends, cycling, hiking, and is dedicated to training for a half marathon.

Valentina Levi
Undergraduate (V.Levi@sms.ed.ac.uk)
Valentina joined the Montagne Lab in January 2025 as a Neuroscience BSc student at the University of Edinburgh. She will be focusing her undergraduate research project on investigating the role of endothelial dysfunction in neurodegeneration and ageing. In her free time, Valentina enjoys pursuing her passions for photography, yoga, and travelling.

Daniela Jaime Garcia
Wellcome Trust Translational Neuroscience PhD Student (dany.jaime-garcia@ed.ac.uk)
Dany joined the Montagne lab in 2021 as a Wellcome Trust Translational Neuroscience PhD student. She is investigating the role of pericytes and endothelial cells in the blood-brain-barrier breakdown observed in small vessel disease and vascular dementia. She completed her undergraduate in psychology and neuroscience at Grinnell College, USA, and subsequently pursued a Masters in Stroke Medicine at the Institute of Neurology, University College London. She has since been a research assistant in clinical studies of small vessel disease with Prof. Joanna Wardlaw at the University of Edinburgh and continues to collaborate with the Wardlaw group for her PhD. In her spare time, Dany loves playing tennis, taking long walks with her dog, cycling, and cooking.

Michael Sewell, Ph.D.
Postdoctoral Researcher (michael.sewell@ed.ac.uk)
Michael joined the Montagne lab in December 2023 as a postdoctoral research fellow after completing his PhD as part of the Wellcome Trust Translational Neuroscience programme in the McColl lab at the University of Edinburgh. During his PhD, he gained extensive experience of using omics approaches to study the roles of microglia in neurodevelopmental disorders. In the Montagne lab, he is interested in using omics approaches to investigate interactions between microglia and cells of the neurovascular unit during blood-brain barrier breakdown. In his free time, Michael enjoys doing and watching anything sports-related, especially formula 1, football, running marathons, and tennis, as well as travelling (having done two round-the-world trips).

Rebecca Billie
MSc Student (R.Billie@sms.ed.ac.uk)
Rebecca joined the Montagne lab in January 2024 as part of her second rotation, Master of Science in Research for Cardiovascular Sciences. In her academic journey she has completed her undergraduates degree from Northern Arizona University (USA), in Bachelors of Science in Biomedical Sciences with two associate degrees in German and Chemistry. Some of her coursework was completed at Tübingen Universität (Germany) as she also served as a global ambassador for the Deutsche-Amerikan Institute (D.A.I.). Previously, she was studying at the University of Edinburgh’s Old Medical school for Masters of Science in Human Anatomy where she transferred to the Queen Medical Research Institute (QMRI) to further specialise in Cardiology. For this MSc rotation, she will be working within Dr. Montagne lab in collaboration with Dr. Mairi Brittan to identify novel targets with a dual regulatory role in small vessel disease in the heart and brain. In her free time, Rebecca advocates and collaborates with the United States and her indigenous, Navajo Nation, governments to help recognise and document her tribal ethnicity, “Alaskan Native/Native American”, within the UK higher educational registry. As she is, by records, the first Native American and woman to be accepted at the UoE since its founding in 1583. She hopes to pursue a career in Cardiothoracic surgery and gain a comprehensive knowledge of medical research in cardiovascular pathology to bring back to her tribal reservations where specialised medical expertise are not accessible.

Emma Clare
MSc Student (E.E.Clare@sms.ed.ac.uk)
Emma joined the Montagne lab in October 2023 as part of her 4-year British Heart Foundation PhD program. She is a graduate of Sport and Exercise Science from Manchester Metropolitan University who then pursued a Master in Cardiovascular Science at the Georg-Agust Universität, Göttingen. For her first rotation of her MSc year, she will be working at the Montagne lab in collaboration with Dr. Mairi Brittan to validate the expression of specific genes at the proteomic level that have been identified using single-cell RNA sequencing. These genes may play an important role in the pathophysiology of the heart-brain axis and are potential targets implicated in multi-systemic small vessel disease.

Caoimhe Lawless O’Beirne
BSc Student (C.Lawless-O’Beirne@sms.ed.ac.uk)
Caoimhe is in her final year of study for a BSc in Medical Sciences. She has a special interest in Neuroscience and will be joining the lab for 12 weeks from January 2023 to undertake her Senior Honours Project. In her free time, Caoimhe enjoys playing hockey, going on runs, and reading.

María Paula Huertas
MSc Student (M.P.Huertas@sms.ed.ac.uk)
María Paula joined the Montagne lab in October 2022 as an MSc Student as part of her PhD. She is a graduate of Molecular Biology from the University of Dundee, from where she then went on to complete a Master’s in Genetics at the Université de Paris Cité. Her interest in clinical and translational research has led her to pursue the 4-Year British Heart foundation PhD programme at the University of Edinburgh. For her first rotation of her MSc year, she will be working at the Montagne lab in collaboration with Dr Mairi Brittan to identify similarities and differences in the endothelial transcriptome in patients with small vessel disease (SVD) in the heart and the brain.

Louis Fizelier
Medical Student (louisfizelier@gmail.com)
Louis spent 2 months in the Montagne lab in July-August 2022. Louis is currently in his 5th year of medical school at the University of Caen Normandy. He has already received hands on training in the departments of Neurology and Neurosurgery at the Centre Hospitalier Universitaire (CHU) of Caen. Louis wanted to gain experience in a Neuroscience wet lab and is currently following a Master Neuroscience programme focused on brain diseases, particularly vascular pathologies. In his free time, Louis plays basketball, enjoys sailing, and loves going to the movies.
