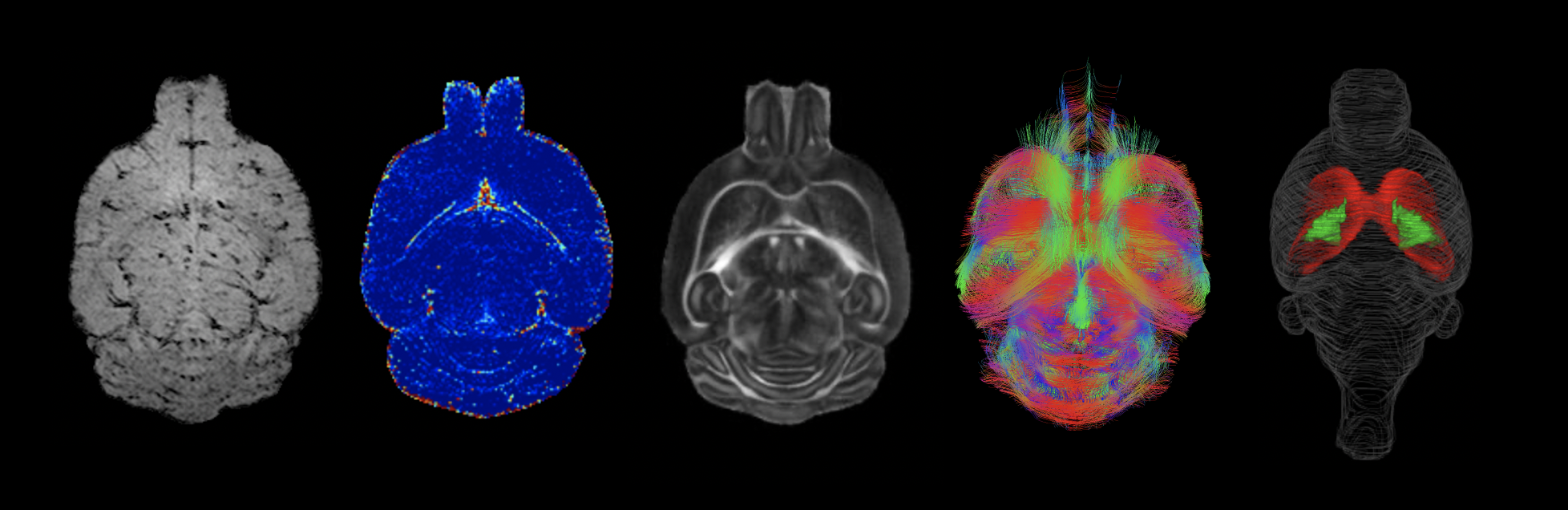
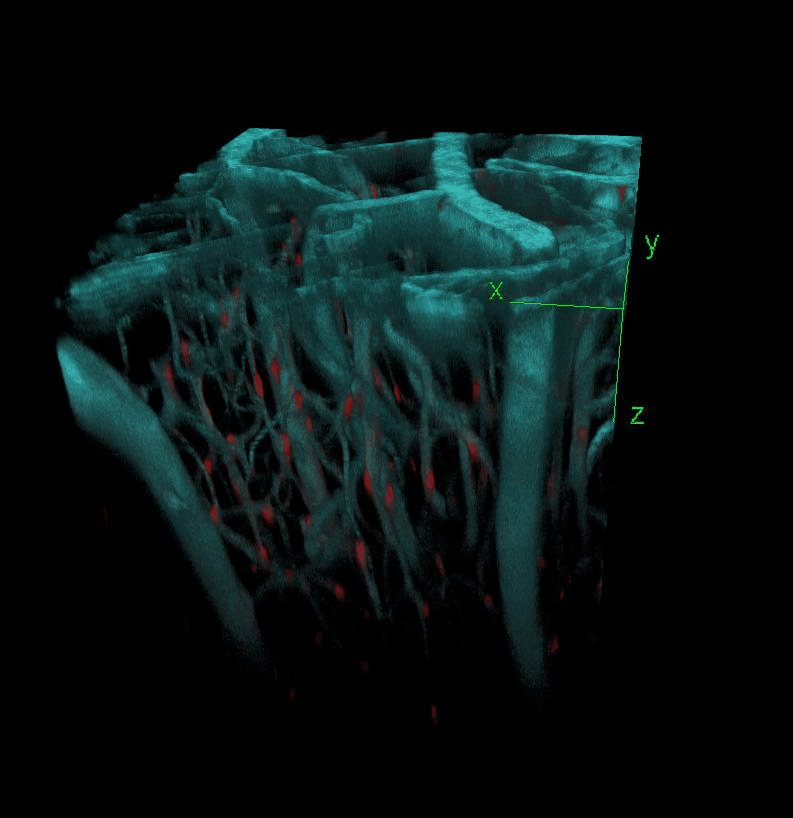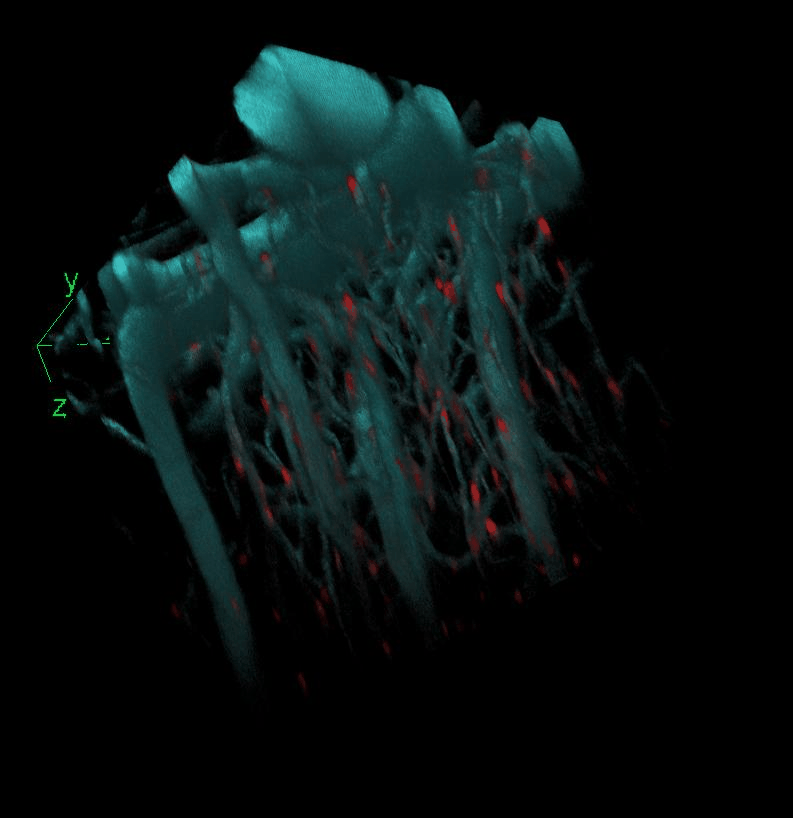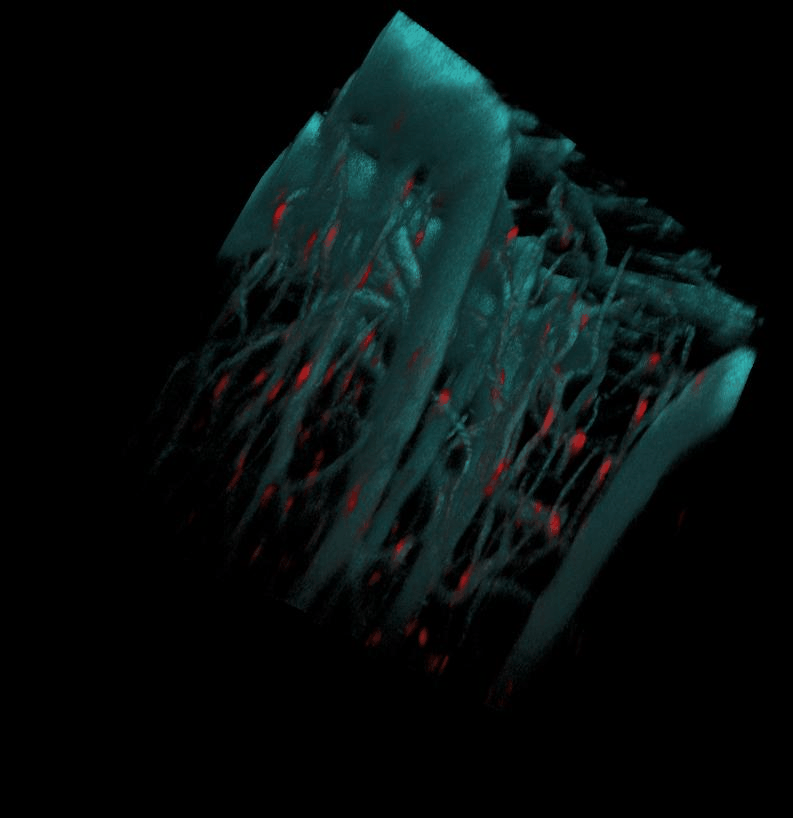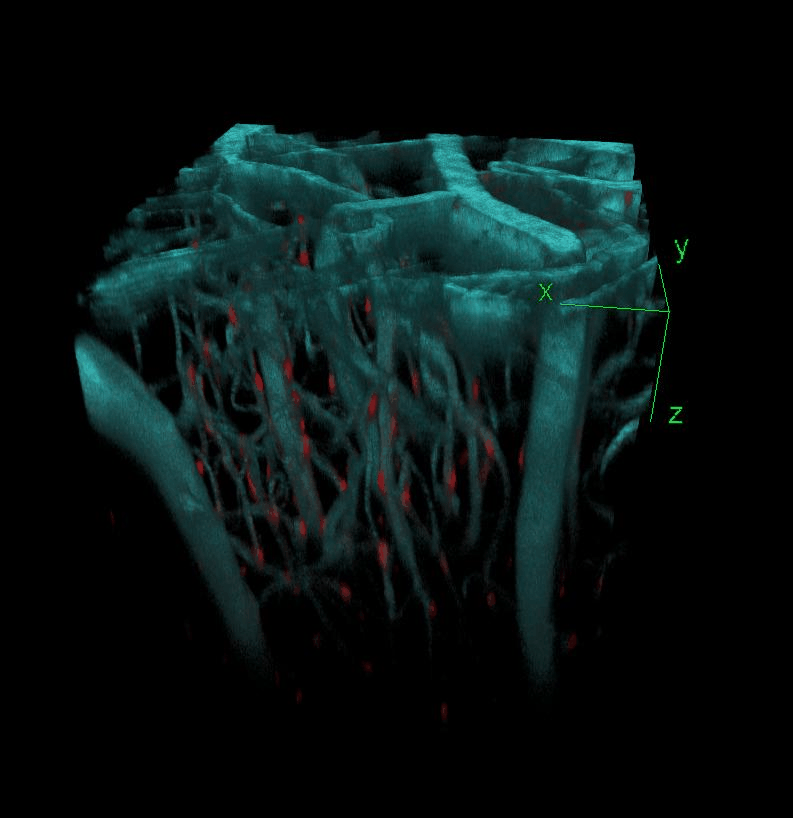
[from left to right: horizontal molecular MRI of endothelial activation, blood-brain barrier permeability map, fractional anisotropy map, 3D-tractography top-view, and volumetric 3D-reconstruction (red, corpus callosum; green, internal capsule)]
A growing body of literature supports the involvement of blood-brain barrier (BBB) dysfunctions in the early stages of brain disorders such as small vessel disease (SVD) and Alzheimer’s disease (AD). In particular, researchers are interested in the role played by pericytes, cells that wrap around endothelial cells and are important for blood vessel formation, BBB maintenance, regulation of immune cell entry into the central nervous system (CNS), and control of brain blood flow.
We have published several human studies providing evidence of a critical role for pericytes in the progression of diseases that cause dementia. We have previously shown that there is an age-dependent BBB breakdown in the human hippocampus, which worsens with mild dementia and correlates with injury to BBB-associated cell pericytes, pericyte dysfunction and BBB breakdown are early biomarkers of human cognitive decline, and BBB breakdown in individuals carrying the ε4 allele of the apolipoprotein E (APOE) gene, a major genetic risk factor for AD, increases with and predicts cognitive impairment.
Our work in animal models has taken these investigations further, demonstrating that pericyte deficiency leads to capillary breakdown initiating early white matter damage, in part due to blood-derived neurotoxic fibrinogen extravascular deposits, and that acute ablation of brain pericytes leads to capillary disruption which triggers a rapid brain circulatory failure and neuronal loss in part due to loss of a pericyte-derived growth factor called pleiotrophin.
With the role of pericytes in brain disorders established, fundamental mechanistic insights must now be uncovered. Questions still remain as to what can cause pericytes to become dysfunctional and ultimately die, and how pericyte loss in turn destabilises the endothelial cells lining the BBB. Greater knowledge in these areas may lead to novel therapeutic targets for cerebrovascular stabilisation in chronic neurodegenerative disease.
Main Objective & Research Goals
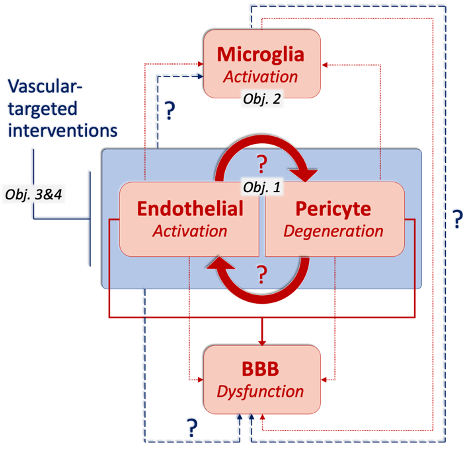
We aim to address these questions by combining advanced molecular approaches with rodent non-invasive imaging, e.g., MRI and PET. We hypothesise that endothelial activation at the BBB is an early trigger for pericyte dysfunction and intend to better understand the consequences of this to the BBB as a whole. Specifically, we will:
1. Assess the impact of modulating both pericyte and endothelial cell functions to define the downstream consequences for the other cell type, and for BBB functions;
2. Determine the role of resident microglia and systemic immune cells in responding to a compromised endothelium-pericyte interplay, and their contribution to BBB integrity;
3. Test BBB preserving interventions in an in vitro platform;
4. Define the impact of vascular-targeted therapeutic interventions for ameliorating vascular, neuronal, and cognitive functions in the context of SVD & AD.
Main Techniques
To achieve these aims, we exploit our expertise in vascular biology combined with the use of magnetic resonance imaging (MRI) in the living mouse brain as the core of our research. We specifically study vascular and neuronal dysfunctions in novel mouse models relevant to SVD and AD. For instance, we apply molecular MRI using iron oxide-based particles to image endothelial activation, contrast MRI using Gadolinium-based contrast agents to study regional BBB permeability and perfusion, multi-shell diffusion techniques to examine microstrutural tissue properties and structural connectivity, to name a few.
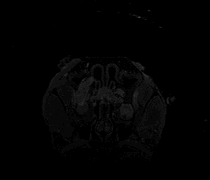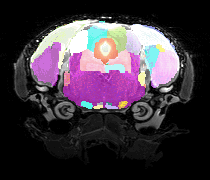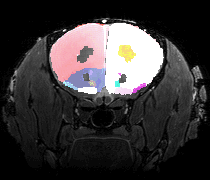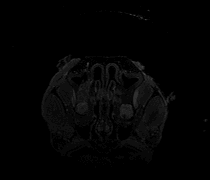
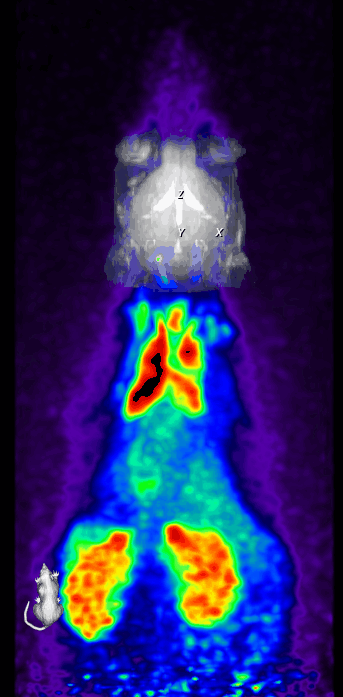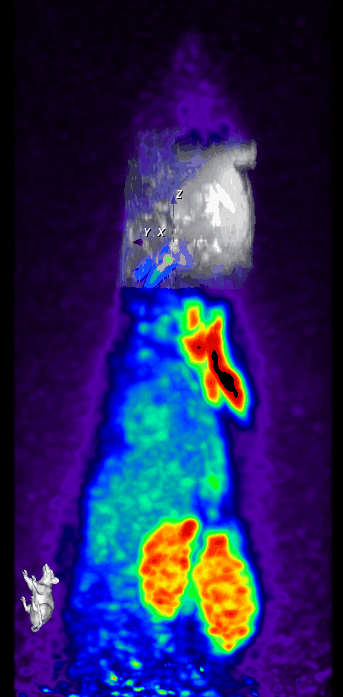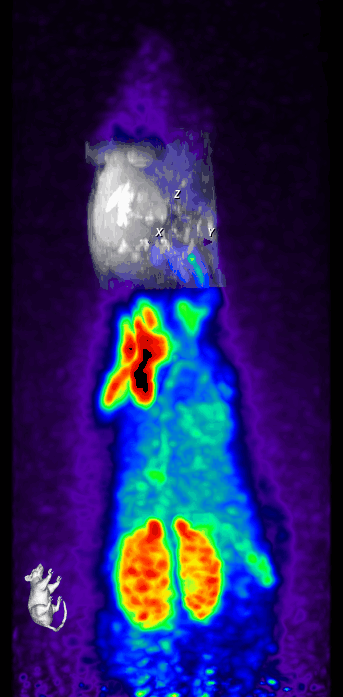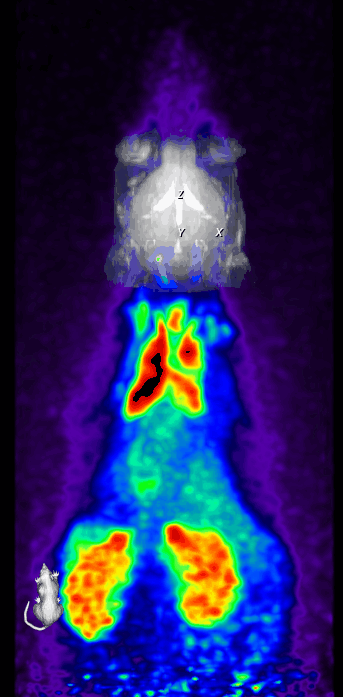
We also perform simultaneous positron emission tomography (PET) which has become an increasingly useful research modality to visualize molecular processes, such as neuroinflammation, amyloid and tau pathologies, and others. For instance, we use [18F]DPA-714, a second-generation PET tracer with improved affinity and selectivity, to visualize inflammation-driven overexpression of TSPO (translocator protein), predominantly expressed in microglia/macrophages, in our models.
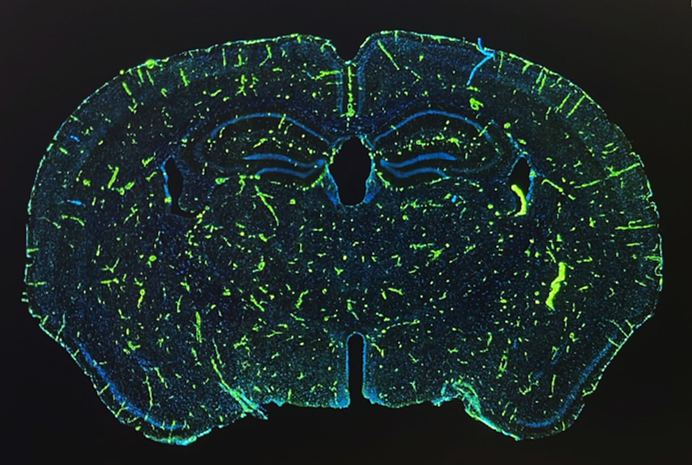
In parallel, two-photon microscopy in the living mouse brain is used to study vascular dysfunctions and the associated immune response through a cranial window. This imaging technique provides important novel insights into cellular and subcellular mechanisms that could not be obtained otherwise in vivo.
In addition to these live imaging techniques and in order to dig further into the biology of BBB functions and dysfunctions in health and disease, my lab is also using in vitro BBB systems, cell-specific genetic manipulation, molecular biology, ‘omics, behavioural assessments, and more.